Technology
- Home
- Aseeds top
- Technology
Our Technology CAR-T cell therapy developed using PiggyBac transposon technology
In 2009, we were the first to report CAR-T cells developed using PiggyBac transposon (PB) technology. Since then, we have worked consistently on the development of CAR-T cells for cancer using PB technology.
What is CAR-T cell therapy?
The cells comprising our body divide every day. During these repeated cell divisions, gene abnormalities are thought to occur, leading to the formation of abnormal cells which can become cancer cells. These abnormal cells are rapidly eliminated by our body’s immune system, in a process known as tumor immunity. Many types of immune cells are involved in tumor immunity. In particular, T lymphocytes play a central role in cancer elimination.
However, when the immune system is broken and becomes unable to eliminate abnormal cells, some remaining abnormal cells progress to become cancer cells. Therefore, the formation of cancer cells can also be considered to be a kind of immune system breakdown.
CAR-T cell therapy is a novel cancer therapy that genetically modifies T lymphocytes that have lost their ability to eliminate cancer cells, to restore their ability to effectively attack cancer cells.
CAR-T cells are produced by genetically modifying T lymphocytes, one type of immune cell, to express an artificial protein called chimeric antigen receptor (CAR). T lymphocytes expressing CAR protein effectively identify cancer cells, and attack them powerfully in a manner that differs from conventional cancer therapies.
Usually, T lymphocytes collected from a patient’s blood are genetically modified and proliferated for several days to several weeks outside of the body, and then administered to the patient for treatment.
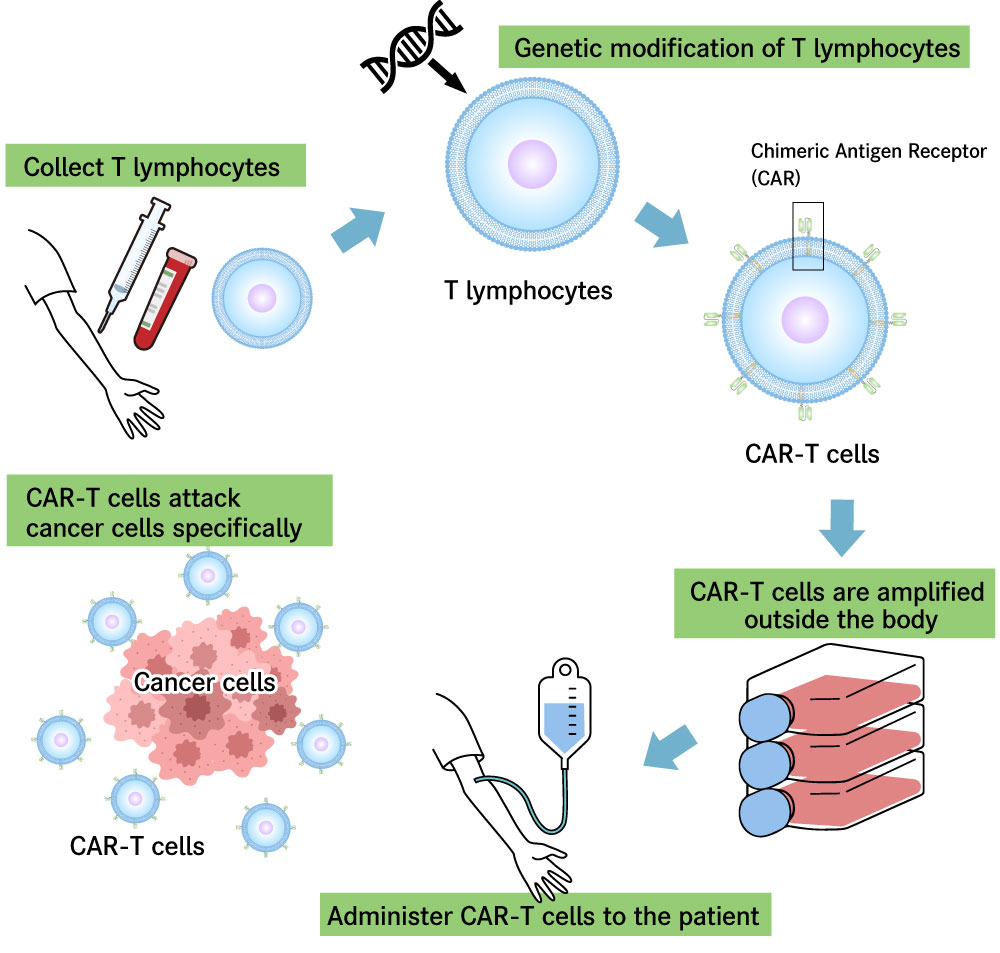
Figure 1: Schematic of CAR-T cell therapy
Current status and problems of CAR-T cell therapy for cancer
As of 2024, CAR-T cell therapy is clinically applied to B-cell tumors, a type of hematologic tumors, and very high therapeutic effects have been reported, even in patients who could not be cured using conventional therapies.
Accordingly, much research has been conducted to make CAR-T cell therapy available for other hematologic tumors and solid tumors. However, it has become apparent that the direct application of conventional CAR-T cells to other tumors is problematic, as the effects of CAR-T cells may be only temporary and not sustained, or serious side effects may emerge. Clinically applicable CAR-T cells have not yet been developed for hematologic tumors other than B-cell tumors or for solid tumors.
Recent research has revealed CAR-T cell characteristics that are closely related to the effects of CAR-T cell therapy. In order to provide sustained efficacy, it is important that CAR-T cells contain a component called “stem cell memory-like T cells.” Thus, a major theme of current research on CAR-T cell development is how to produce CAR-T cells containing memory T cells.
Our technology – producing stem cell memory-like CAR-T cells using PiggyBac transposon technology
Most CAR-T cell products currently used for treatment are manufactured by genetically modifying T cells using “viral vectors.” However, it has been revealed that T cells differentiate and lose their memory function in the process of genetic modification by virus vectors.
In this regard, we have succeeded in developing CAR-T cells that are less susceptible to a loss of efficacy due to immune exhaustion, which has been recognized as a major problem, by genetically modifying T cells using PB technology, without using viral vectors.
In addition, another reason why CAR-T cells produced by PB technology are likely to retain their memory function is that the CAR gene is predominantly transfected into memory T cells when producing CAR-T cells using our technology.
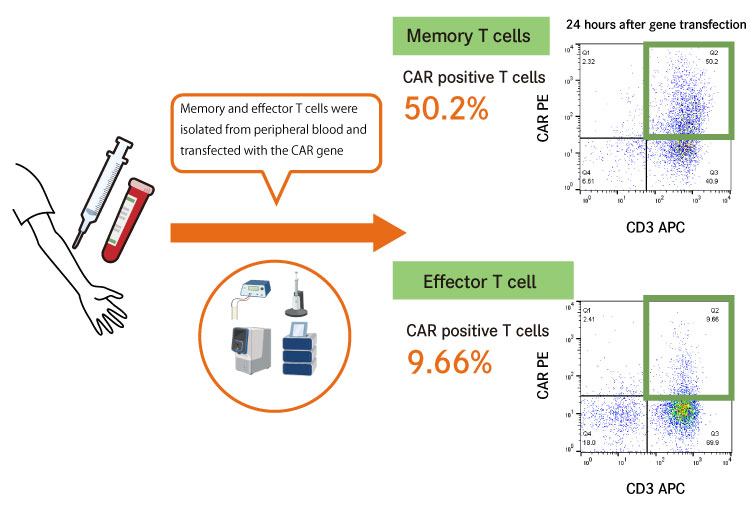
Figure 2: The CAR gene is predominantly transfected into memory T cells
In combination with our cell amplification technology, we also succeeded in predominantly amplifying CAR gene-transfected memory T cells, and ultimately in producing ideal CAR-T cells that are characterized by less differentiation and less immune exhaustion.
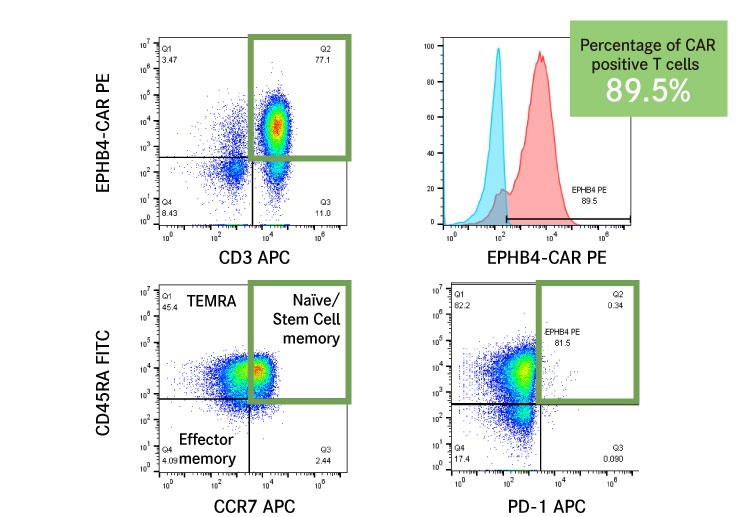

Currently, most CAR-T cells are manufactured using viral vectors. However, differentiation due to possible activation of T cells and immune exhaustion are problems that are usually seen in these CAR-T cells. By producing CAR-T cells with higher quality using our technology, we can create CAR-T cells which are capable of providing sustained efficacy.
In addition, this non-viral vector gene modification is expected to make it easier to deliver CAR-T cells to patients, because it can significantly reduce manufacturing costs and employs simpler manufacturing processes.
Challenges in solid cancer treatment – attacking multiple targets simultaneously
Although the development of CAR-T cell therapy for solid tumors has been desired, CAR-T cell products with high therapeutic efficacy for solid tumors have not yet been developed. One of reasons for this situation is that the expression of the cancer antigens to be targeted by CAR-T cells is not uniform in solid tumors, and targeting a single cancer antigen is not sufficient to eliminate all cancer cells.
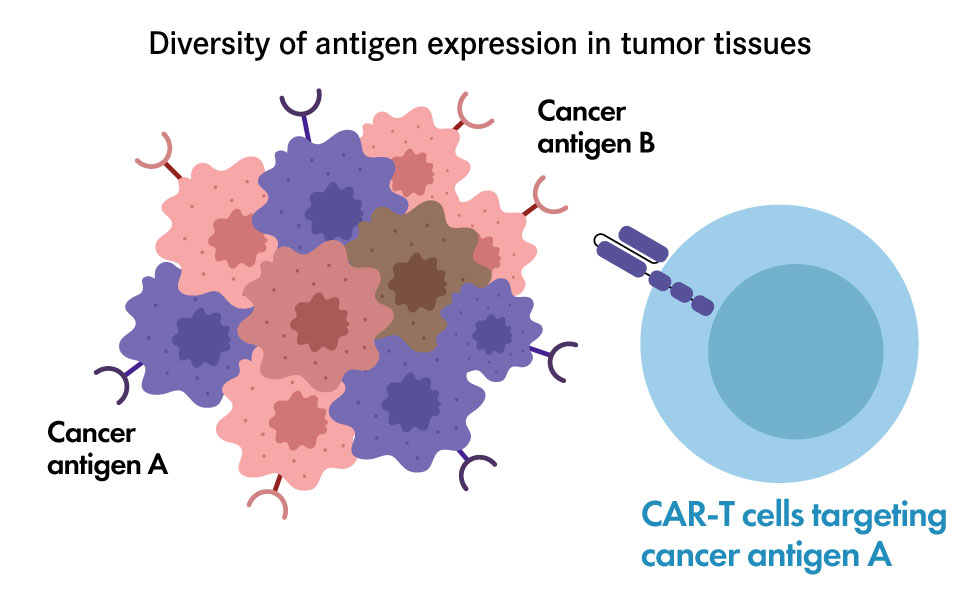
- Heterogeneous antigen expression
- Antigen-negative relapse
- ”Targeting multiple tumor antigens”
concept can overcome antigen heterogeneity
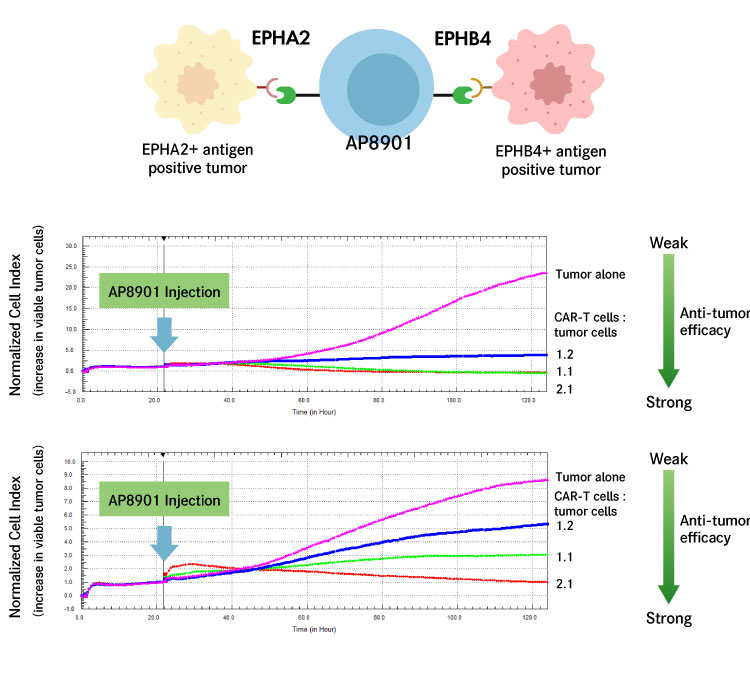
We have developed CAR-T cells that can bind to both of two cancer antigens A and B, by modifying the CAR protein that binds specifically to a cancer antigen.
We are developing CAR-T cells (AP8901) that can simultaneously recognize cancer antigens EPHB4 and EPHA2, which are expressed on many cancer cells, and attack cancer cells expressing EPHB4 and those expressing EPHA2 at the same time.
This simultaneous recognition of multiple cancer antigens makes it possible to efficiently attack cancer tissues, which are populations of diverse cells, and prevent the recurrence of cancer.